| Identification | Back Directory | [Name]
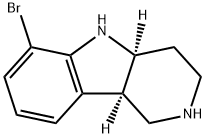
(4aS,9bR)-6-bromo-2,3,4,4a,5,9b-hexahydro-1H-pyrido[4,3-b]indole | [CAS]
1059630-07-7 | [Synonyms]
EOS-60906
Lumateperone Intermediate
6-bromo-2,3,4,4a(S),5,9b(R)-hexahydropyrido[4,3-b]indole
(4aS,9bR)-6-bromo-2,3,4,4a,5,9b-hexahydro-1H-pyrido[4,3-b]indole
1H-Pyrido[4,3-b]indole, 6-bromo-2,3,4,4a,5,9b-hexahydro-, (4aS,9bR)-
N-[2-[benzyl(thiophen-2-ylmethyl)amino]-2-oxoethyl]-2,4-dimethoxy-N-(2-morpholin-4-ylethyl)benzamide | [Molecular Formula]
C11H13BrN2 | [MDL Number]
MFCD30663123 | [MOL File]
1059630-07-7.mol | [Molecular Weight]
253.14 |
| Chemical Properties | Back Directory | [Boiling point ]
338.4±42.0 °C(Predicted) | [density ]
1.416±0.06 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Inert atmosphere,2-8°C | [pka]
10.02±0.20(Predicted) | [Appearance]
Light yellow to yellow Solid | [InChI]
InChI=1S/C11H13BrN2/c12-9-3-1-2-7-8-6-13-5-4-10(8)14-11(7)9/h1-3,8,10,13-14H,4-6H2/t8-,10-/m0/s1 | [InChIKey]
LPBJHSPFSUDRSY-WPRPVWTQSA-N | [SMILES]
N1C2=C(C=CC=C2Br)[C@]2([H])CNCC[C@]12[H] |
| Hazard Information | Back Directory | [Synthesis]
Example 2: Preparation of [4aS,9bR]-6-bromo-2,3,4,4a,5,9b-hexahydro-1H-pyrido[4,3-b]indole: 6-bromo-2,3,4,5-tetrahydro-1H-pyrido[4,3-b]indole hydrochloride (8.48 mmol) was mixed with trifluoroacetic acid (630 mL) and triethylsilane (172 mL). The reaction mixture was stirred at room temperature for 19 h under nitrogen protection. Excess trifluoroacetic acid and triethylsilane were removed under vacuum. Hexane (550 mL) was added to the residue, stirred at room temperature for 1 hour and decanted. Hexane (250 mL) was added repeatedly and stirred for 1 hour and decanted. The residual oily mass was adjusted to pH=10 by addition of 2N sodium hydroxide solution and subsequently extracted with dichloromethane. The organic layers were combined, washed with brine and dried over anhydrous sodium sulfate. Enantioseparation of [4aS,9bR]-6-bromo-2,3,4,4a,5,9b-hexahydro-1H-pyrido[4,3-b]indole can be accomplished by dissolving the racemate 6-bromo-2,3,4,4a,5,9b-hexahydro-1H-pyrido[4,3-b]indole (8 g, 31.6 mmol) in methanol (160 mL) in a 50 °C oil bath in batches (R)-mandelic acid (4.8 g, 31.6 mmol) was added. After stirring for a few minutes, ether (80 mL) was added dropwise, cooled to room temperature, and filtered to obtain a white precipitate (R-mandelate, 3.7 g). HPLC analysis showed >99% ee. The filtrate was concentrated, treated with 1N sodium hydroxide (100 mL), and extracted with dichloromethane (2 x 50 mL). The organic layers were combined, washed with brine, dried over sodium sulfate and concentrated to give an oil (5.59 g). It was dissolved in methanol (90 mL), (S)-(+)-mandelic acid (3.53 g, 23.2 mmol) was added in batches, stirred for a few minutes and then ether (45 mL) was added dropwise, cooled to room temperature, and filtered to give a white precipitate (S-mandelate, 4.19 g).HPLC analysis showed >99% ee. R-mandelate: [α]D25 = -98.1; S-mandelate acid salt: [α]D25 = +102 (solvent: DMSO). Alternative methods allow splitting in a mixture of methanol and tert-butyl methyl ether (MTBE). Alternatively, the racemate 6-bromo-2,3,4,4a,5,9b-hexahydro-1H-pyrido[4,3-b]indole (9.61 g, 38.0 mmol) was dissolved in methanol (190 mL), and (S)-(+)-mandelic acid (5.78 g, 38.0 mmol) was added in batches with stirring for a few minutes and then added dropwise with ether (95 mL) and cooled to room temperature. Filtration yielded a white precipitate (S-mandelate, 4 g). HPLC analysis showed >99% ee. Large-scale enantiomeric separation can be achieved by dissolving racemic 6-bromo-2,3,4,4a,5,9b-hexahydro-1H-pyrido[4,3-b]indole (1710 g, 6.21 mol) in methanol (24 L), heating to 40 °C, and adding a one-time dose of (R)-(-)- mandelic acid (944 g, 6.2 mol), cooled and added MTBE (13 L), aged with stirring at room temperature for 30-40 h. A white to off-white precipitate was obtained by filtration (Int-2 R-mandelate, 580 g, 23%). Chiral HPLC showed >99% ee. The filtrate was concentrated, alkalized and extracted with dichloromethane, dried and concentrated to give the free base (~1150 g). It was dissolved in methanol (17 L), (S)-(+)-mandelic acid (692 g, 4.55 mol) was added, MTBE (8.5 L) was added, and aged at room temperature for 30-40 h. S-mandelate (828 g, 33%) was obtained by filtration. Chiral HPLC showed >99% ee. R-mandelate: [α]D25 = -98.1; S-mandelate: [α]D25 = +102 (solvent: DMSO). Chiral HPLC conditions: ChiralPak AD-H column, 250 × 4.6 mm, 30% isopropanol/hexane (containing 0.1% diethylamine), flow rate 0.8 mL/min, UV detection 254 nm. | [References]
[1] Patent: WO2008/112280, 2008, A1. Location in patent: Page/Page column 82-85
[2] Drugs of the Future, 2015, vol. 40, # 10, p. 643 - 650 |
|
|





![6-bromo-2,3,4,4a,5,9b-hexahydro-1H-pyrido[4,3-b]indole](/CAS/20180703/GIF/1059630-12-4.gif)
