| Identification | Back Directory | [Name]
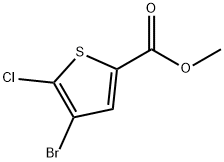
Methyl 4-broMo-5-chlorothiophene-2-carboxylate | [CAS]
1047630-72-7 | [Synonyms]
4-broMo-5-chlorothiophene-2-carboxylate
methyl 4-bromo-5-chloro-2-thiophenecarboxyl
Methyl 4-broMo-5-chlorothiophene-2-carboxylate
methyl 4-bromo-5-chloro-2-thiophenecarboxylate
4-Bromo-5-chloro-2-thiophenecarboxylic acid methyl ester
2-Thiophenecarboxylic acid, 4-bromo-5-chloro-, methyl ester | [Molecular Formula]
C6H4BrClO2S | [MOL File]
1047630-72-7.mol | [Molecular Weight]
255.52 |
| Chemical Properties | Back Directory | [Boiling point ]
293.9±35.0 °C(Predicted) | [density ]
1.762±0.06 g/cm3(Predicted) | [storage temp. ]
under inert gas (nitrogen or Argon) at 2–8 °C | [Appearance]
White to off-white Solid |
| Hazard Information | Back Directory | [Synthesis]
General procedure for the synthesis of methyl 5-chloro-4-bromothiophene-2-carboxylate from methyl 5-chlorothiophene-2-carboxylate: To a 1 L round bottom flask was added aluminum chloride (11.32 g, 85 mmol) and methyl 5-chlorothiophene-2-carboxylate (10 g, 56.6 mmol) dissolved in chloroform (250 mL). Bromine (4.08 mL, 79 mmol) was added slowly and dropwise over 10 min. The reaction mixture was stirred at 25 °C for 6 h. The light orange reaction solution was washed with saturated sodium bicarbonate solution. The organic layer was dried over anhydrous sodium sulfate, filtered and concentrated. The residue was purified by silica gel column chromatography [n-hexane/ethyl acetate, 9:1] to give the target product (12 g, 80% yield) as a white solid.LC-MS (ESI) m/z 256 ([M+H]+). | [References]
[1] Patent: WO2008/98104, 2008, A1. Location in patent: Page/Page column 188
[2] Drugs of the Future, 2014, vol. 39, # 8, p. 541 - 546 |
|
|