| Identification | Back Directory | [Name]
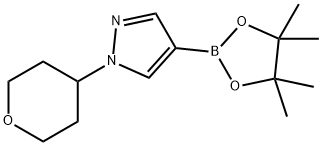
1-(tetrahydro-2H-pyran-4-yl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole | [CAS]
1040377-03-4 | [Synonyms]
1-(oxan-4-yl)-4
1-(4-Tetrahydropyranyl)-1H-pyrazole-4-boronic acid pinacol ester
1-(Tetrahydro-2H-pyran-4-yl)pyrazole-4-boronic acid pinacol ester
1-(Tetrahydropyran-4-yl)-1H-pyrazole-4-boronic acid pinacol ester
1-(oxan-4-yl)-4-(tetraMethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole
1-(oxan-4-yl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrazole
1-(Tetrahydro-2H-pyran-4-yl)-1H-pyrazole-4-boronic Acid Pinacol Ester
1-(Tetrahydropyran-4-yl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole
1-(tetrahydro-2H-pyran-4-yl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole
1H-Pyrazole, 1-(tetrahydro-2H-pyran-4-yl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-
3-hydroxy-2,3-dimethylbutan-2-yl hydrogen (1-(tetrahydro-2H-pyran-4-yl)-1H-pyrazol-4-yl)boronate | [Molecular Formula]
C14H23BN2O3 | [MDL Number]
MFCD12033229 | [MOL File]
1040377-03-4.mol | [Molecular Weight]
278 |
| Chemical Properties | Back Directory | [Melting point ]
113.0 to 117.0 °C | [Boiling point ]
413.8±35.0 °C(Predicted) | [density ]
1.16 | [storage temp. ]
Inert atmosphere,Store in freezer, under -20°C | [form ]
powder to crystal | [pka]
2.70±0.19(Predicted) | [color ]
White to Light yellow |
| Hazard Information | Back Directory | [Chemical Properties]
1-(tetrahydro-2H-pyran-4-yl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole Appearance is white to light yellow powder to crystalline, melting point 113.0 ~117.0 °C. | [Uses]
1-(tetrahydro-2H-pyran-4-yl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole is an important pharmaceutical intermediate compound used in the preparation of crizotinib. | [Synthesis]
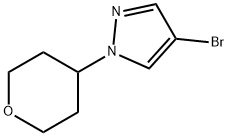
A reaction mixture was prepared from 4-bromo-1-(tetrahydro-2H-pyran-4-yl)-1H-pyrazole (1.0 g, 4.33 mmol) and pinacol ester of bis(boronic acid) (1.32 g, 5.19 mmol) in 10 mL DMF. Potassium acetate (1.27 g, 12.98 mmol) and 1,1'-bis(diphenylphosphino)ferrocene-palladium(II) dichloride dichloromethane complex (177 mg, 0.22 mmol) were added sequentially and the reaction was carried out under argon protection. The reaction mixture was stirred at 80 °C for 10 hours. After completion of the reaction, the reaction solution was diluted with 40 mL of water and subsequently extracted with ethyl acetate (30 mL x 3). The organic phases were combined, washed sequentially with water (30 mL x 3) and brine, dried over anhydrous Na2SO4 and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography (ethyl acetate/petroleum ether, 1/4) to afford 1-(tetrahydropyran-4-yl)-1H-pyrazole-4-boronic acid pinacol ester (39) as a white solid in 68% yield. The product was characterized by NMR (300 MHz, CDCl3): δ 7.80 (s, 1H), 7.75 (s, 1H), 4.42-4.31 (m, 1H), 4.12-4.07 (m, 2H), 3.57-3.49 (m, 2H), 2.13-1.99 (m, 4H), 1.32 (s, 12H). | [References]
[1] Bioorganic and Medicinal Chemistry, 2012, vol. 20, # 17, p. 5169 - 5180
[2] Bioorganic and Medicinal Chemistry, 2013, vol. 21, # 21, p. 6804 - 6820
[3] Patent: WO2015/81257, 2015, A2. Location in patent: Page/Page column 84-85
[4] Patent: WO2011/79804, 2011, A1. Location in patent: Page/Page column 41
[5] Patent: US2012/245178, 2012, A1. Location in patent: Page/Page column 25 |
|
|