| Identification | Back Directory | [Name]
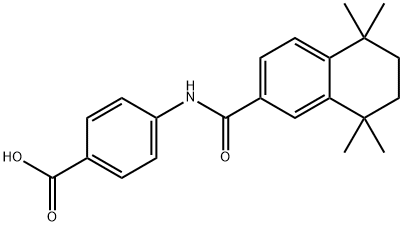
4-[(5,6,7,8-TETRAHYDRO-5,5,8,8-TETRAMETHYL-2-NAPHTHALENYL)CARBOXAMIDO]BENZOIC ACID | [CAS]
102121-60-8 | [Synonyms]
cd336
AM 580
CS-1051
l)amino-
ro40-6055
NSC 608001
AM580 (CD336)
AM-580;AM 580;CD336; NSC608001; RO 40-6055
4-(1,1,4,4-Tetramethyltetralin-6-ylcarbonylamino)benzoic acid
4-[(5,6,7,8-TETRAHYDRO-5,5,8,8-TETRAMETHYL-2-NAPHTHALENYL)CA...
4-[(5,5,8,8-tetramethyl-6,7-dihydronaphthalene-2-carbonyl)amino]benzoic acid
4-((5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)carbonyl)aminobenz
benzoicacid,4-((5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)carbony
4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydronaphthalene-2-carboxamido)benzoic acid
2-Naphthalenecarboxamide,5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-N-3-quinolinyl-
4-[(5,6,7,8-TETRAHYDRO-5,5,8,8-TETRAMETHYL-2-NAPHTHALENYL)CARBOXAMIDO]BENZOIC ACID
4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydronaphthalene-2-ylcarbonylamino)benzoic acid
4-[[(5,6,7,8-Tetrahydro-5,5,8,8-tetramethylnaphthalen)-2-yl]carbonylamino]benzoic acid
4-[[(5,6,7,8-Tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)carbonyl]amino]benzoic acid
4-{[(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-yl)carbonyl]amino}benzoic acid
Benzoic acid,4-[[(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)carbonyl]amino]- | [Molecular Formula]
C22H25NO3 | [MDL Number]
MFCD00673916 | [MOL File]
102121-60-8.mol | [Molecular Weight]
351.44 |
| Chemical Properties | Back Directory | [Melting point ]
265-267℃ | [Boiling point ]
485.24°C (rough estimate) | [density ]
1.154 | [refractive index ]
1.5614 (estimate) | [storage temp. ]
−20°C
| [solubility ]
Soluble in DMSO (20mg/ml) or ethanol (10mg/ml). | [form ]
White to off-white solid. | [pka]
4.28±0.10(Predicted) | [color ]
Off-white | [Stability:]
Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| Hazard Information | Back Directory | [Description]
AM-580 (102121-60-8) is a retinoic acid analog acting as a RARα agonist (Kd = 8 nM).1 Induces or blocks differentiation depending on cell type and environment.2,3 In combination with CHIR-99021 it induces differentiation of human induced pluripotent stem cells (iPSCs) into intermediate mesoderm (80% induction rate in 5 days) capable of forming kidney structures.4 AM-580 exhibits anti-angiogenic activity in vivo, and induces differentiation of acute promyelocytic leukemia cells.5,6 | [Uses]
Retinoic acid analog which acts as a selective RARα agonist with no affinity for RXRs. Induces differentiation in leukemia cell lines and inhibits angiogenesis in chick embryos. | [Definition]
ChEBI: An amidobenzoic acid obtained by formal condensation of the carboxy group of (5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-yl)benzoic acid with the anilino group of 4-aminobenzoic acid. A selective RARalpha agonist. | [General Description]
AM580 is a retinobenzoic derivative and a RAR-α (retinoic acid receptor-α) agonist. It stimulates the maturation of granulocytes in NB4 promyelocytic leukemia cell line and APL (acute promyelocytic leukemia) blasts. AM580 suppresses endometrial cancer cell proliferation. | [Biological Activity]
An analog of retinoic acid that acts as a selective RAR α agonist (EC 50 values are 0.3, 8.6 and 13 nM for RAR α , RAR β and RAR γ respectively). Significantly induces IL-4, IL-5 and IL-13 and inhibits IL-12 and IFN γ synthesis, and induces cell differentiation with over 7 times the activity of retinoic acid in vitro . | [Synthesis]
To a 25 mL round-bottomed flask equipped with a reflux condenser tube was added methyl 4-(5,6,7,8-tetrahydro-5,5,8,8-tetramethylnaphthalene-2-carboxamido)benzoate (0.200 g, 0.547 mmol), methanol (10 mL) and potassium hydroxide (0.122 g, 2.18 mmol). The reaction mixture was stirred under reflux conditions for 16 h and subsequently evaporated to dryness. The residue was partitioned between ethyl acetate and deionized water and adjusted to acidic pH with 6 N hydrochloric acid. the organic layer was separated, washed sequentially with deionized water, saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated by rotary evaporation to afford 4-(5,6,7,8-tetrahydro-5,5,8,8-tetramethylnaphthalene-2-carboxamido)benzoic acid as a white solid (0.151 g, 78% yield ). Molecular weight: 351.43; melting point: 265°C; Rf value: 0.3 (ethyl acetate:cyclohexane=50:50).1H NMR (CDCl3, δ): 1.32 (s, 6H, 2×CH3), 1.34 (s, 6H, 2×CH3), 1.73 (s, 4H, 2×CH2), 7.43 (d, 1H, J=8.53 Hz. ArH), 7.57 (d, 1H, J=8.25 Hz, J=1.98 Hz, ArH), 7.78 (d, 2H, J=8.74 Hz, ArH), 7.88 (d, 1H, J=8.74 Hz, ArH), 7.92 (s, 1H, NH), 8.13 (d, 2H, J=8.70 Hz, ArH), and no observed carboxylic acid proton signal.MS-ESI: m/z (relative intensity) 352.1 ([M+H]+, 100).HPLC analysis (Method A, detection wavelength 254 nm): retention time = 6.66 min, peak area = 96.3%. | [storage]
Store at RT | [References]
Bernard et al. (1992), Identification of synthetic retinoids with selectivity for human nuclear retinoic acid receptor gamma; Biochem. Biophys. Res. Commun. 186 977
Safonova et al. (1994), Fatty acids and retinoids act synergistically on adipose cell differentiation; Biochem. Biophys. Res. Commun. 204 498
Shibuyua et al. (2005), Retinoic acid is a potential negative regulator for differentiation of human periodontal ligament cells; J. Periodontal Res. 40 432
Araoka et al. (2014), Efficient and rapid induction of human iPSCs/ESCs into nephrogenic intermediate mesoderm using small molecule-based differentiation methods; PLoS One. 9 e84881
Oikawa et al. (1993), Three novel synthetic retinoids, Re 80, Am 580 and Am 80, all exhibit anti-angiogenic activity in vivo; Eur. J. Pharmacol.249 113
Gianni et al. (1996), AM580, s stable benzoic acid derivative of retinoic acid has powerful and selective cyto-differentiating effects on acute promyelocytic leukemia cells; Blood 87 1520 |
|
|




![Benzoic acid, 4-[[(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)carbonyl]amino]-, methyl ester](/CAS/20210305/GIF/102121-59-5.gif)
